
In the UK, across the EU and globally, a strong momentum is building to improve the lives of people living with rare diseases. This includes the UK Rare Diseases Framework, Eurordis’s #30millionreasons for European action on rare diseases campaign and the global community’s call for a UN resolution on the issue. Patient groups are pressing for better access to care and treatments, and for a greater understanding of the corrosive and all-encompassing impact conditions have on their lives as members of society.
In the UK, around 3.5 million people have to cope with the debilitating effects of rare diseases, according to the Department of Health and Social Care. The vast majority wait years for an accurate diagnosis and then struggle to access effective treatment - if it even exists.
Promising policy initiatives have launched that support the progress of research into and access to rare disease therapies. The current focus on addressing health inequalities is encouraging. However, implementation will be key and consistency between policies the litmus test. For instance, how can the UK be a leader in genomics and still lag its European partners for newborn screening? How can health inequalities within the rare community be fully addressed if medicines approval criteria are often failing to consider fully the implications on all affected?
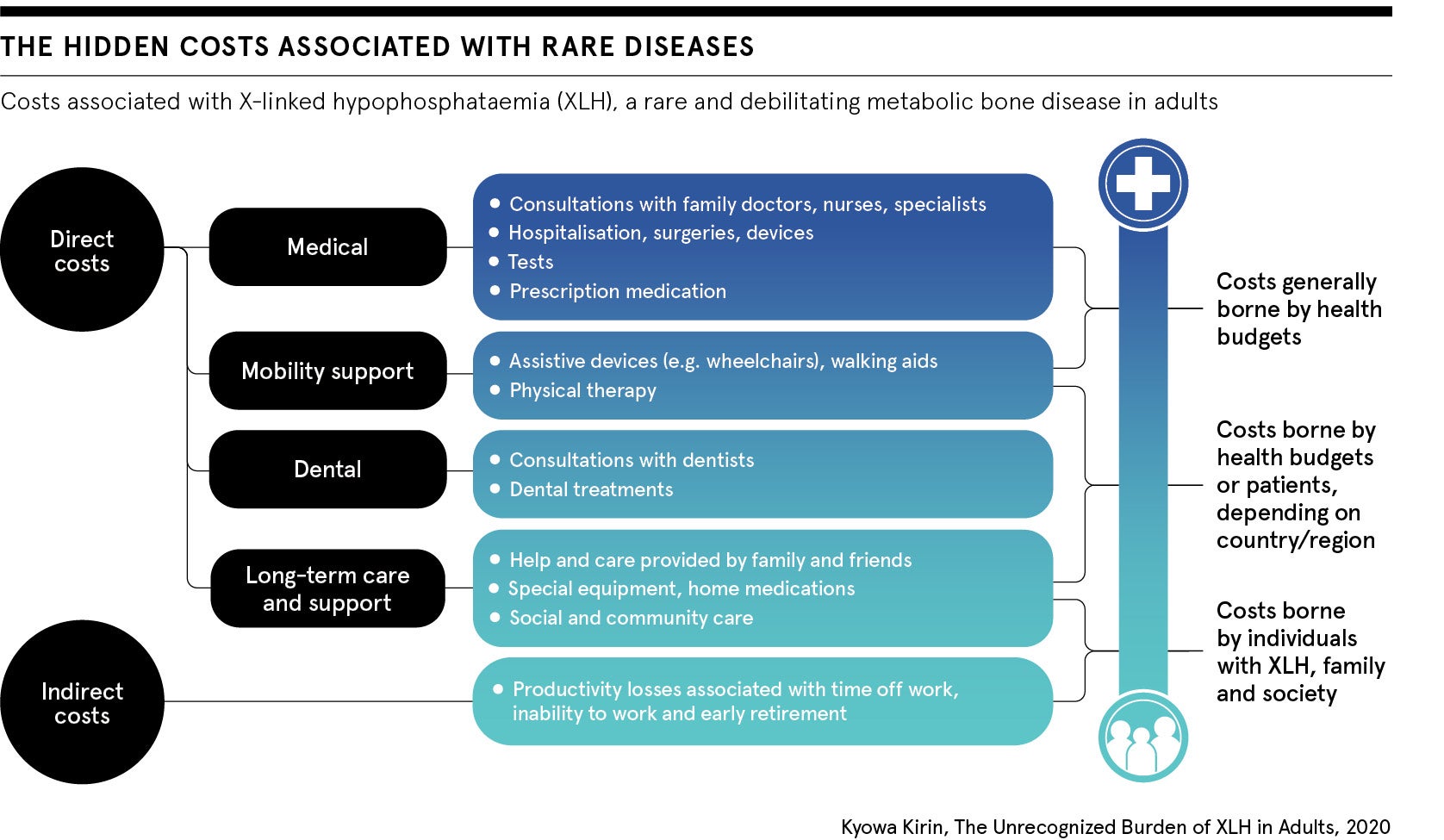
“There is so much uncertainty for people living with rare conditions,” says Victoria Hayes, director of public affairs, Northern Cluster, for Kyowa Kirin, a Japan-based global specialty pharmaceutical company involved in rare diseases. “Inequality exists all along their journey from diagnosis to access to treatment and care, with hidden costs to society.”
The National Institute for Health and Care Excellence (otherwise known as NICE) has just carried out a consultation on its evaluation methods to support the ambition of the NHS to provide high-quality care that offers good value to patients and to the NHS.
“Kyowa Kirin believes the proposed changes – to be confirmed when the outcome of the consultation is made public – are a step in the right direction. We welcome the work being done but want to see more because people with rare diseases are hugely disadvantaged,” says Richard Johnson, Kyowa Kirin general manager, Northern Cluster.
Research in this area poses specific challenges. The small number of patients and the fewer treatment options mean, for example, that to ethically recruit in a clinical trial finding the right comparator is arduous.

“Most importantly, all of us need to have the right mindset to meaningfully listen and engage with people living with rare conditions. Rare conditions can come with the risk of downward social mobility. People can be stifled in their career aspirations, while their personal relationships and family life are affected. Research suggests that, in some cases, there is a downward social spiral for families where the disease is hereditary,” says Johnson.
The UK Rare Disease Framework sets out the basis for each of the four UK nations to draft their action plans, expected to be published shortly.
“The devil will be in the detail; we hope these plans will be an important step forward. Access to multi-disciplinary teams that can see the whole person will be crucial,” says Hayes. “Like the patient groups we work with, we want to see the uncertainty taken out of the system and greater flexibility introduced when considering rare conditions in terms of clinical trials and drug approval. At the end of the day what matters is people and their lives; the full picture not just the medical notes.”
For more information on Kyowa Kirin please visit https://international.kyowa-kirin.com/uk/
Promoted by Kyowa Kirin

In the UK, across the EU and globally, a strong momentum is building to improve the lives of people living with rare diseases. This includes the UK Rare Diseases Framework, Eurordis’s #30millionreasons for European action on rare diseases campaign and the global community’s call for a UN resolution on the issue. Patient groups are pressing for better access to care and treatments, and for a greater understanding of the corrosive and all-encompassing impact conditions have on their lives as members of society.
In the UK, around 3.5 million people have to cope with the debilitating effects of rare diseases, according to the Department of Health and Social Care. The vast majority wait years for an accurate diagnosis and then struggle to access effective treatment - if it even exists.
Promising policy initiatives have launched that support the progress of research into and access to rare disease therapies. The current focus on addressing health inequalities is encouraging. However, implementation will be key and consistency between policies the litmus test. For instance, how can the UK be a leader in genomics and still lag its European partners for newborn screening? How can health inequalities within the rare community be fully addressed if medicines approval criteria are often failing to consider fully the implications on all affected?

