
A structured approach to deploy the driving force of data can help companies energise their business and create a landscape where innovations such as artificial intelligence (AI) and predictive analytics move closer.
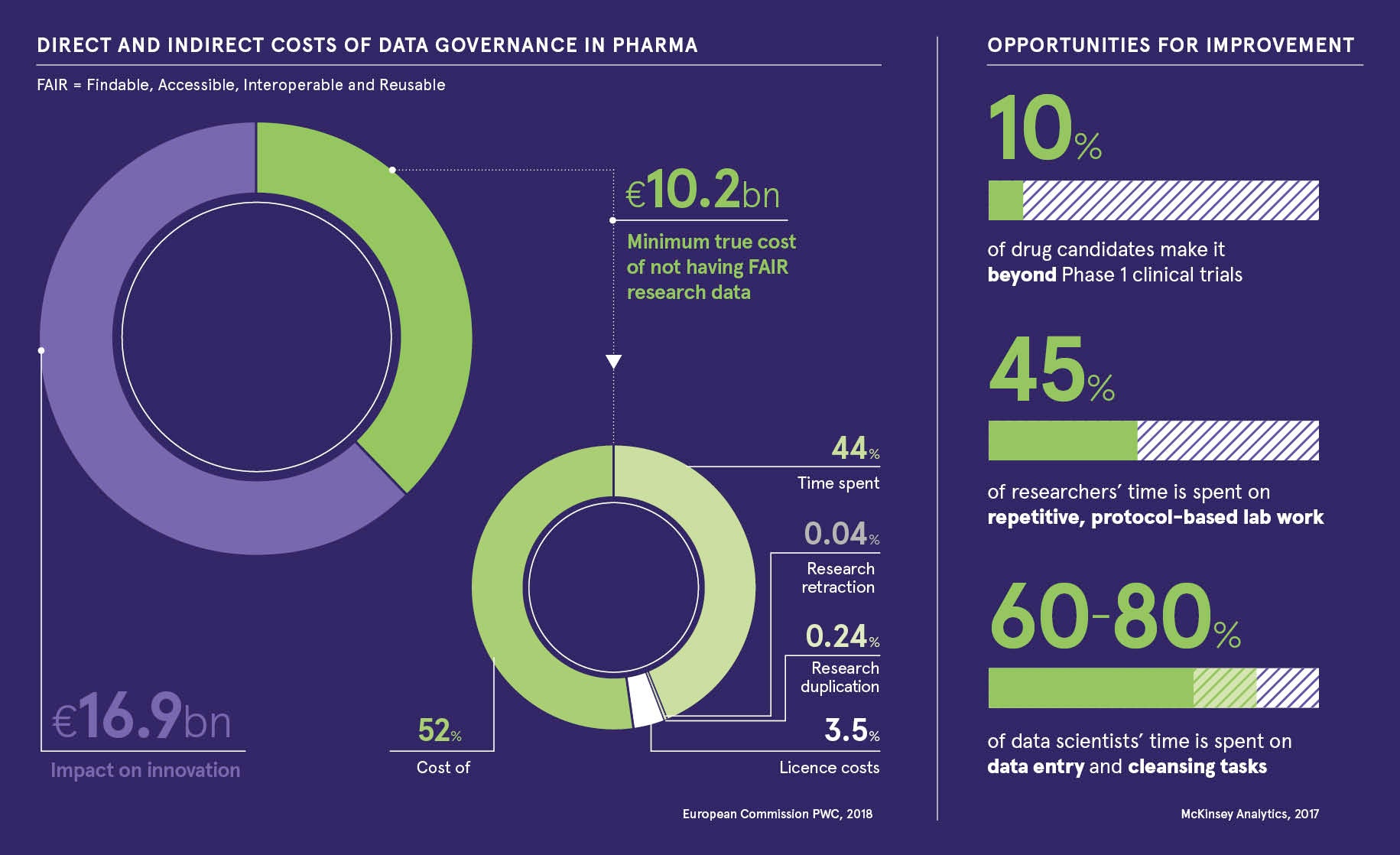
Research has shown that failures to realise digital innovation cost the industry €16.9 billion1 a year, while labour-intensive procedures corrode the morale of scientists and compromise their ability to discover and develop new therapies.
“Getting your digital approach wrong has a massive impact on a company’s bottom line,” says Dr Haydn Boehm, head of commercial marketing at Connected Lab, a part of Merck, which is dedicated to unlocking the potential of data across the entire pharma industry value chain.
“Scientists are spending up to 60 per cent of their time on data entry and cleansing tasks when they should be concentrating on the science. But there are easy steps we can take to liberate their time and deliver value-based change, rather than viewing AI as something that will magically transform everything.”
Analysts at McKinsey & Company have characterised digital in research and development as the “$100-billion opportunity”2 with its potential to rewrite the current script of single drug development costing $2.6 billion3, while return on investment continues to fall.
Boehm answers questions about the crucial steps needed to harness data to drive improvements across every aspect of pharma, from inventory to security and regulation to staff retention.
Why do pharma companies need to examine how they view and use digital?
Everyone views AI as a panacea, but the real value is in drilling down into how your data can be used in your company. There could be many easy opportunities to leverage digital technologies and services to unlock the full potential of your business and increase process efficiencies. But if your data is not captured efficiently, or is not readily accessible to the people who need it, you are never going to be able to create data-driven business models.
What are the first steps to making data work for a company?
It is important to recognise processes that involve a lot of human labour and motion waste, or unnecessary movements, which can be a significant element of how your data is captured, stored and used. Addressing this lays the foundation for utilising more AI and getting measurable benefits from it. Some companies are hiring scientists simply to cleanse data but, if you optimise data capture and input, you can employ those scientists to do what they are trained for and what they want to do.
What is the potential from enhanced digital practice?
It can have a transformative influence where it matters, reducing costs, improving productivity, increasing capacity and speed of delivery, which has a huge impact on customer satisfaction. This enhances brand reputation and company equity. It also allows companies to optimise resources and the integrity of data, which is a fundamental springboard to greater use of AI across the organisation. The challenge is to analyse your approach to data and calculate where digital will be most effective.
Why is it important to have good data?
If your data is clean and trustworthy, you can achieve so much more scientifically and structurally across the company. It is also vital for security and compliance, which are very important factors. Having data that cannot be shared with external partners, or even internally, hampers development, and pursuing the industry-standard practice of producing trial and safety data on PDFs can cause issues with regulatory authorities. The US Food and Drug Administration and European Medicines Agency are moving away from paper reports and into digital, so companies need to synchronise with their requirements or run the risk of censure. Having data that is not compliant with FAIR (findability, accessibility, interoperability and reusability) principles is another issue that hits the bottom line and a PwC report estimated it costs the European economy €10.2 billion a year in delays and lost opportunities.
Can it improve drug development?
This is exactly what it does. Enhancing and harmonising processes and reducing motion waste creates more time and space to innovate and pursue discovery. The targeted use of digital technology can help deliver better predictors of what would be a successful molecule. Better data governance means you can collaborate on more productive terms and also interrogate historical data to generate new leads and speed up discovery processes. There are so many opportunities going to waste because the data is not clean or reusable, or it is difficult to locate and share. At the core of this is the scientist and anchoring them down with data-cleansing tasks and multiple transcriptions of reports is counter-productive. They need to be spending 100 per cent of their time problem-solving and driving scientific programmes forward. They should not be shackled when digital resource management can free them to do their job.
What are the downsides of not addressing data?
The impact of lost opportunities is immense and it means companies lose out to competitors on many fronts. Contracts are lost, confidence is affected, staff retention becomes an issue and, ultimately, brand reputation suffers. All these negatively impact the balance sheet. It is key to look at areas where utilising digital technology can make a difference, such as producing reports or responding to regulatory affairs, which can be a massive human burden, or having scientists spending 45-60 per cent of their time cleansing data.
1 Cost of not having FAIR research data, PwC EU Services March – 2018
2 Digital in R&D: The $100 billion opportunity, McKinsey Analytics 2017
3 Tufts Center for the Study of Drug Development, Briefing: Cost of Developing a New Drug, November 18, 2014
Promoted by Merck

A structured approach to deploy the driving force of data can help companies energise their business and create a landscape where innovations such as artificial intelligence (AI) and predictive analytics move closer.
Research has shown that failures to realise digital innovation cost the industry €16.9 billion1 a year, while labour-intensive procedures corrode the morale of scientists and compromise their ability to discover and develop new therapies.
“Getting your digital approach wrong has a massive impact on a company’s bottom line,” says Dr Haydn Boehm, head of commercial marketing at Connected Lab, a part of Merck, which is dedicated to unlocking the potential of data across the entire pharma industry value chain.

