
In an era when developing a medicine takes an average £1.2 billion over 12 years, taking on innovative projects could seem a risky strategy. But chief scientific officer Dr David Altshuler explains how the Vertex strategy has allowed the company to forge a string of innovative successes, including breakthrough treatments for cystic fibrosis (CF). The leading geneticist and pioneer of human genome projects tells how the company, which has a research laboratory in Oxford, is tackling serious diseases with unmet needs and seemingly intractable complexities.
What is Vertex’s approach to developing therapies?
We believe the greatest value to society, patients and other stakeholders is to discover and develop new medicines that transform the lives of people with serious diseases. That is our true north. We are not looking to make incremental advances or to treat downstream symptoms; we want to strike at the heart of disease.
How does Vertex decide which disease conditions to research?
We start with a laser-like focus on serious diseases for which there is no transformative therapy. Next, we look for a deep insight into the human causal disease biology, compelling evidence about the root cause in people, not just in the laboratory or in a fruit fly. We look for breakthroughs in the science of therapeutics. In many of the diseases we’re working in, there may not have been existing tools or approaches to treating the underlying cause of disease, and our scientists have had to invent or partner to bring new processes, techniques and therapeutic strategies.
Isn’t that a risky starting point?
Actually, we believe that a strategy focused on understanding the human causal biology is more likely to result in breakthroughs for patients. Take CF as an example. Thirty years ago, the cause of the disease was identified as a mutation in a gene that is responsible for transporting chloride, effectively salt, across cell membranes. The need was clear, but there was no existing technology to restore chloride transport. So others focused on treating infections downstream or to thin the mucus. These efforts helped patients and deserve praise but, crucially, did not address the underlying cause. Vertex scientists said ‘well, we may be able to create a new type of medicine that can restore the function of the mutant protein’. And, amazingly, they were able to succeed in that goal.
How did that work in CF?
As background, the majority of oral medicines act by blocking the activity of the target. But with CF our scientists had the unprecedented idea to create a medicine that acts on a mutant protein to coax it to work more normally. It was unclear how to do this, but over 20 years of work that is exactly what they achieved.
How does that differ from other approaches?
Many companies use what’s called a shots-on-goal approach. That is they try many different approaches based on laboratory models, hoping one of them will help patients. Our view is to focus our resources and innovation on those relatively few outstanding scientific opportunities where all the pieces line up: a serious disease with limited treatment options, a deep insight into human biology and the right biomarkers, and a new therapeutic approach. We believe these opportunities deserve more attention because that’s where we can make the greatest impact for patients right now.
Can you give a concrete example?
Because we select targets that are well validated, our clinical development strategy is to bring multiple therapeutic candidates into the clinic and investigate them in parallel. By studying multiple candidate medicines at the same time, we can mitigate risk of compound-specific failures and this enables us to select the best possible candidate based on patient data rather than laboratory data alone. In this way, the compound selected to advance into large, phase-III trials is intended to have the best profile we can achieve. This approach requires conviction on the target and greater upfront investment, but enables more rapid progress and lower risk of expensive late-stage failure, all with the goal of bringing the best medicines to patients as quickly and safely as possible.
What is in the Vertex pipeline?
We are pursuing a number of exciting projects that fit our strategy, including research for patients with sickle cell disease, beta thalassemia, type-1 diabetes and alpha 1 antitrypsin deficiency disease. We’ve assembled a robust toolkit of technologies and capabilities, including cell and genetic therapy platforms that will allow us to directly address these diseases from multiple angles. We are confident our strategy has great promise for patients and, if we are able to help patients, everything else will follow.
What is the role of patient advocacy groups in your R&D strategy?
Appropriate engagement with patient organisations is key to help us understand the lives and daily experiences of the people they represent. Patient communities have insights that are crucial to inform the development of a medicine, at all stages of the process. A good example of this is working with patient groups to continually improve the way we run clinical trials; patient-centric clinical trials will ultimately enhance the effectiveness and speed of drug discovery. In the case of CF, it has helped develop the first medicine to treat the underlying cause of CF and we aim to follow this approach in all the diseases whether it is sickle cell disease, beta thalassemia, alpha 1 antitrypsin deficiency disease or others.
How important is R&D to Vertex successes?
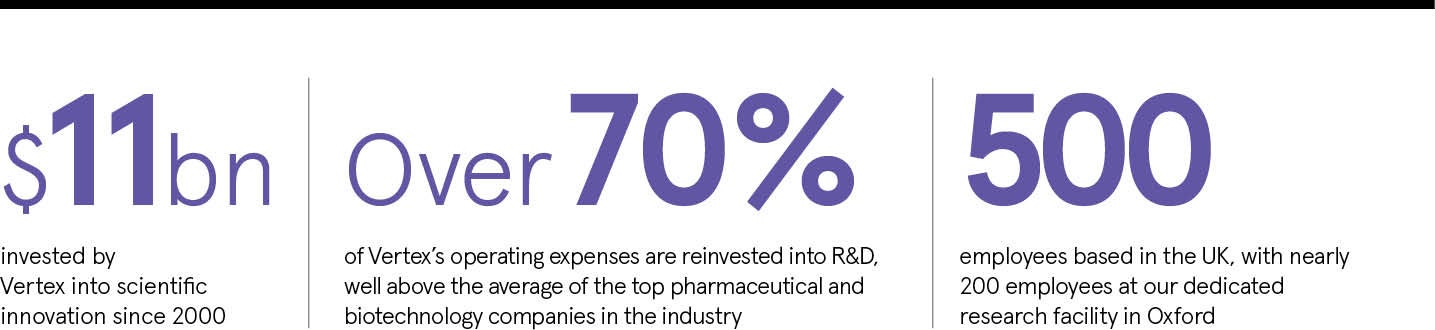
Our strategy is to invest in scientific innovations that break open or create new possibilities to treat serious diseases. And we put our money where our mouth is: we spend more than 70 per cent of our operating expenses on research and development (R&D) and three out of five Vertex employees are dedicated to R&D.
What does the pursuit of COVID-19 vaccines say about the biopharmaceutical industry?
The progress towards a vaccine has been astounding and makes you very proud of everyone involved: companies, academic scientists and doctors, the regulators and governments. Because of advances in genomics and therapeutics, the underlying cause of COVID was discovered in weeks instead of years, and multiple therapeutics and vaccines have been advanced all within 2020. This experience reminds us that when we tackle serious diseases with urgency, focus and collaboration, we can move forward in a manner that previously would have been seen as impossible.
For more information please visit vrtxpharma.co.uk
Date of Prep: November 2020 UK-00-2000014
Promoted by Vertex

In an era when developing a medicine takes an average £1.2 billion over 12 years, taking on innovative projects could seem a risky strategy. But chief scientific officer Dr David Altshuler explains how the Vertex strategy has allowed the company to forge a string of innovative successes, including breakthrough treatments for cystic fibrosis (CF). The leading geneticist and pioneer of human genome projects tells how the company, which has a research laboratory in Oxford, is tackling serious diseases with unmet needs and seemingly intractable complexities.
What is Vertex’s approach to developing therapies?
We believe the greatest value to society, patients and other stakeholders is to discover and develop new medicines that transform the lives of people with serious diseases. That is our true north. We are not looking to make incremental advances or to treat downstream symptoms; we want to strike at the heart of disease.

