SPONSORED BY Medidata
The quest to produce a viable coronavirus vaccine continues and the aftershocks of the pandemic will rattle through healthcare for several months to come, but technology is highlighting its ability to play an increasing role in drug development.
Strategic application of mobile technology, artificial intelligence (AI) and machine-learning is having a growing influence on clinical research and its multifaceted capabilities underscore its potential to be a meaningful tool in clinical studies to help bring treatments to patients faster.
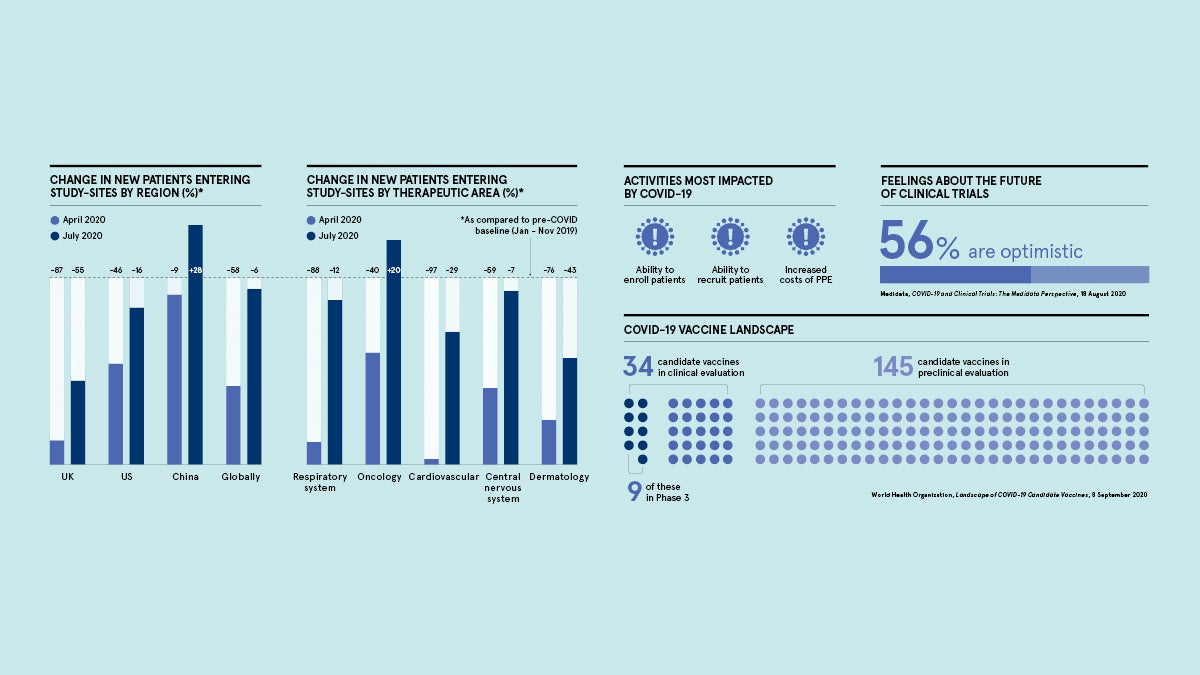
A white paper titled COVID-19 and Clinical Trials, conducted by Medidata, records that 60 per cent of the industry are currently optimistic about the future of clinical trials. In April, Medidata saw a 58 per cent decrease in patients entering clinical trials globally and an 87 per cent drop in the UK specifically. Fast forward to July and these numbers are down to 6 per cent and 55 per cent respectively. So the optimism is warranted.
“COVID-19 has obviously had a significant impact with a reduction in the number of trials and patients enrolling in trials, but that is starting to bounce back,” says Fareed Melhem, who leads Acorn AI Labs, Medidata’s data science innovation lab. “The optimism comes from a general belief that we will get past the pandemic. And there’s optimism that this period has accelerated the use of technology and the lessons we are learning around how to simplify trials for patients during the pandemic will endure post-COVID.”
Medidata has pioneered software solutions to support the running of clinical trials for 20 years and has generated data from 20,000 trials involving more than six million patients. The company has been able to develop a comprehensive suite of services that deliver improved data collection and trial management, as well as the ability to pull out patterns and provide insights by deploying cutting-edge AI and data analytics.
Healthcare regulators, who are responsible for approving new drugs and treatments and are thoroughly involved in the clinical trial process, have shown flexibility through the pandemic without compromising patient safety.
The long-term view across the industry is that technology will be a dominant force in the future of drug development. There’s a clear cost and time benefit, but perhaps more importantly it minimises the burden on patients. It helps remove logistical barriers, such as travel and taking time off work, so patients are encouraged to participate and remain in trials and be more involved in its outcomes.
“There has been a challenge to maintain data flow during the pandemic, but it has also really accelerated the conversations we’ve been having around the application of technology in healthcare over the past five, ten years,” says Paul O’Donohoe, Medidata’s scientific lead, eCOA (electronic clinical outcome assessment) and mobile health.
“We’ve been speaking about remote data capture using smartphones and tablets for several years, so we’re not necessarily doing anything new as we respond to this situation. What is new is just how rapidly we’ve seen people jump in on the technology, as well as the flexibility we’re seeing from the regulators.
“It has created an opportunity to demonstrate that these technologies work very well and that we can run clinical trials largely remotely and use technology to capture good quality data from patients, while offering them a better experience. The success of sustaining clinical trials with large virtual elements will only encourage its use from an early stage in future trials.”
Around 30 per cent of trials can be conducted completely virtually and there are always elements that can be conducted remotely. By continuously collecting patient data, from a wearable for example, you get a more complete picture of the data and holistic view of the patient, compared to singular check-ins at a clinic. Patient platforms and portals, such as newly launched myMedidata, enable patients to participate more fully in clinical trials and offers more patient-centric solutions.
“We’re getting feedback from patients as we build and develop our technology to make sure it’s technology they want to use when they are enrolled in a trial,” says O’Donohoe. “We have patients at the centre of what we do and are keen to explore anything we can do to make it as positive an experience as possible. We are minimising unnecessary visits and doing as much as possible at home without losing those key touchpoints with the investigator or the clinical staff.”
The imperative is to drive high-quality data that creates valuable, actionable clinical outcomes. Melhem adds: “We support a large proportion of the trials running globally and the data we generate contributes to a lot of positive change across the industry. Acorn AI was launched with the specific intention of getting more insight from the data so we can design better trials for patients and for companies, accelerate enrolment and monitor the execution of trials in real time to keep them on track. Our goal is really to drive a lot more efficiency in the clinical trial process.”
For example, the ability to use synthetic control arms, utilising historic data to mimic the control group, means more patients are on the active drug rather than placebo, hugely benefiting research companies as well as patients.
Melhem concludes: “Healthcare faces a lot of challenges in the future, not just from the pandemic and its aftermath, and our insights can help create more precise treatments with more targeted trials so the promise of what they can deliver for individual patients is much greater.
“We will learn a lot from the pandemic, but one thing it has clearly shown us is technology in clinical trials is vital and will play an integral role in our ability to get treatments to patients faster.”
We will learn a lot from the pandemic, but one thing it has clearly shown us is technology in clinical trials is vital and will play an integral role in our ability to get treatments to patients faster
For more information please visit medidata.com