Rapidly ageing populations living with increased co-morbidities and stretched financial resources are placing huge strains on healthcare systems.
Traditional drug development is slow, unpredictable and cloaked with prohibitive costs, but the digitalisation of clinical trials, use of data and application of new technologies is now providing accelerated pathways to new treatments and energising scientific potential.
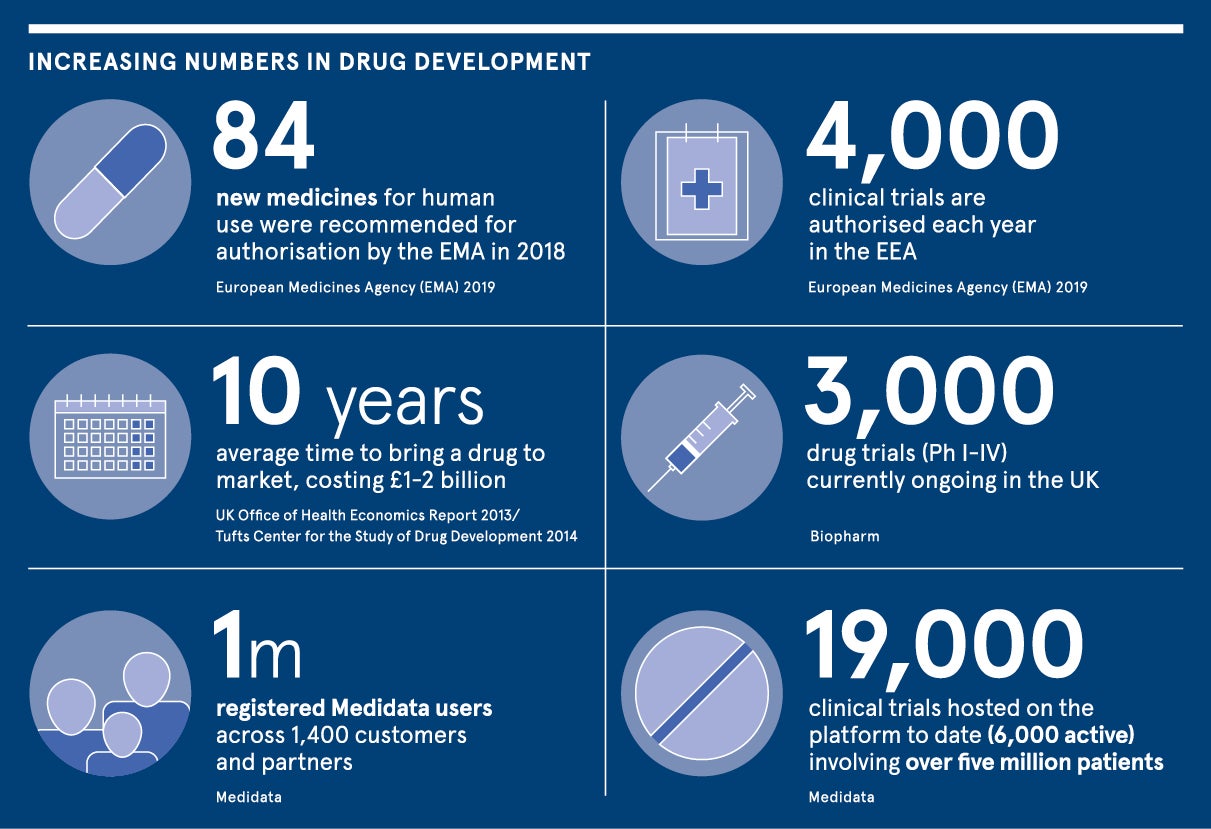
It takes an average of ten years and between £1-2 billion to get a drug to market. By collecting, analysing and harnessing clinical and patient data more efficiently, we can drastically reduce time and cost while also providing enhanced scope to find novel therapies.
“We are starting to see what is possible and there is a great future ahead,” says Christian Hebenstreit, senior vice president and general manager of Medidata, EMEA.
Medidata, a Dassault Systèmes company, is the leading software provider for managing and running clinical trials and streamlining drug development. The company’s innovative platform has powered 19,000 trials involving more than five million patients.
“Our science and technology is generating a huge amount of data that is then translated into value in the quest to get the right drug to the right patient at the right time,” says Mr Hebenstreit.
“We are seeing tremendous progress in a number of therapeutic areas, especially as we are looking into rarer diseases and different areas of cancer. This is thanks to the power of data and the ability to digitalise the drug development process and manage a clinical trial from one streamlined platform, from trial design and patient recruitment to regulatory submission and approval.”
Recruiting suitable patients for clinical trials is a ponderous process and retaining them can be an issue that massively impacts the outcome. Digitalisation has helped to identify the right patients, made it easier for them to take part, and has also opened up a wider and more diverse patient population.
A promising 28 per cent of clinical trials can now be completely virtualised with data collected and interrogated to provide the key findings across efficacy and safety, while often revealing fresh areas of research and patient benefit.
“We work in a highly regulated environment for very good reasons as we are dealing with people’s lives and some testing will not change,” says Mr Hebenstreit. “But finding the right patients to fit the criteria of a trial is one of the biggest issues for companies. It is complicated and can take months to recruit, particularly as many patients might face geographical, cultural or financial barriers.
“Opening it up to home-monitoring for patients, who may live 200 miles away or even in a different country, is a game-changer. Patient recruitment, enrolment and retention is suddenly more efficient. We are also able to replicate control groups for clinicians using historical data, automate processes, remove duplicative data entry and spot errors immediately. It saves time, reduces costs and is ethically very positive as more patients can participate in the trials and therefore get access to new drugs and better treatments much sooner.”
Digitalisation is also acting as a research catalyst promoting collaboration across big pharma and networks of research institutions and startups that now have an open landscape to test novel ideas once restricted by silo mentalities and prohibitive project costs.
Regulatory authorities are fully behind digitalisation and we are seeing more collaboration between the public and private sectors within life sciences than ever before. Medidata regularly engages with regulators around the world with common goals of improving patient experiences and clinical trial outcomes.
The German government, for example, has recently taken it a stage further by including digitalisation in clinical trials in new legislation designed to advance drug development and improve healthcare.
“The industry continues to push for more innovative solutions and the use of data science, big data and new technologies. And the encouragement from regulators is strong because they can see the opportunities coming from digitalisation,” says Mr Hebenstreit.
“It benefits providers, physicians, hospitals, pharmaceutical and biotech companies and, most importantly, will help us to improve the lives of patients.
“Europe, and especially the UK, is well placed to make the best of digitalisation, especially as we explore the phenomenal potential from new technology, artificial intelligence and machine-learning. The change is already here. There is a lot of work to be done, but the future is exciting.”
For more information please visit www.medidata.com