
The way clinical trials are conducted has not traditionally favoured the rare disease community. Typically conducted at medical centres in large cities, where specialist clinicians are located, participation in clinical research has long been a challenge. Patients with rare diseases are, by definition, limited – although collectively, many are affected (approximately 5% of the world population) – and often experience debilitating symptoms which make clinical trial participation challenging.
Almost 40% of rare disease patients travel over 60 miles for healthcare. Clinical research sites are often much further away. Even if they can reach clinics, participation can be long and tiring. Participants must adhere to strict visit schedules over the treatment period – anywhere from six months to many years. Each visit requires substantially more testing and time than a normal doctor’s visit. Time required in a research study adds a commitment burden to participants and their families and is a key barrier to participation.
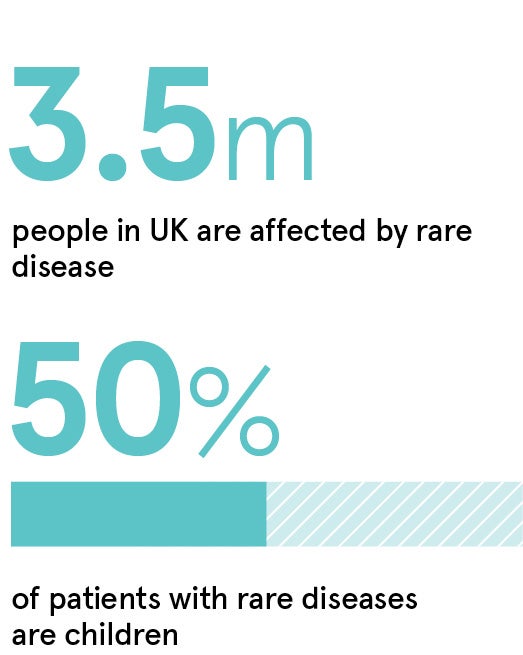
“Rare disease research has historically been very challenging, but new solutions are now available to make participation easier” says Joyce Moore, head of rare disease solutions at THREAD, a leading provider of decentralised clinical trial technology that has supported over 50 global rare disease trials. “The number of patients available with a rare disease is inherently small, and requirements for frequent long-distance travel to research clinics may discourage many otherwise motivated patients. When you consider that 50% of rare disease patients are children, this adds additional complexities.”
The solution, to many in medical research, is clear: decentralisation. Advances in healthcare technologies like telemedicine, mobile surveys and digital communications, mean many clinical trial procedures can take place in a patient’s home and clinical assessments can be reported securely online. While the technology to enable this experience has been available, the biopharmaceutical industry and regulators (MHRA, FDA, EMA) have been slow to adopt these new solutions.
That is until COVID-19. The pandemic required the biopharmaceutical industry and regulators to support new ways of engaging participants. The combined effort ensured that crucial clinical trials could continue to take place during national lockdowns. The result was a tipping point.
“While the biopharmaceutical industry was developing new vaccines, there remained a significant effort and need to maintain safety and data integrity in all clinical trials. Patient safety was of the utmost importance,” Moore says. “It was an important moment for rare disease sufferers as regulators supported the biopharmaceutical industry to incorporate decentralised elements in studies and by doing so increase inclusion, patient choice and flexibility.”
THREAD is playing a key role in this shift to decentralised studies. THREAD helps biopharma and life science organisations capture data from participants and sites remotely during, between, and in lieu of in-clinic visits. The company’s mission seeks to make trials five times more inclusive and 30% more efficient through one comprehensive technology platform. Driven by this mission, THREAD has become a reliable partner for an industry looking to help people with rare diseases.
“Our experience over the last six years taught us how important it is to give people with rare diseases more opportunities for their care. For a large rare disease registry, we implemented new data dashboards that provide real-time information to participants so they could compare their experience with others. This, along with the platform’s ease-of-use and features, led to a 50% increase in registry participation and successful patient recruitment across 12 new clinical trials,” notes Moore.
DCT approaches like these are already helping researchers conduct more efficient studies and bring new therapies to market faster.
“There is still a large unmet need for new rare disease treatments in the UK, where 3.5 million people are affected,” Moore says. “THREAD has a large rare disease portfolio, including trials in the UK. By speeding up the clinical trial process, and making it more accessible, we can get vital rare disease treatments to those that need them quicker.”
For more information visit threadresearch.com
Promoted by THREAD

The way clinical trials are conducted has not traditionally favoured the rare disease community. Typically conducted at medical centres in large cities, where specialist clinicians are located, participation in clinical research has long been a challenge. Patients with rare diseases are, by definition, limited – although collectively, many are affected (approximately 5% of the world population) – and often experience debilitating symptoms which make clinical trial participation challenging.
Almost 40% of rare disease patients travel over 60 miles for healthcare. Clinical research sites are often much further away. Even if they can reach clinics, participation can be long and tiring. Participants must adhere to strict visit schedules over the treatment period – anywhere from six months to many years. Each visit requires substantially more testing and time than a normal doctor’s visit. Time required in a research study adds a commitment burden to participants and their families and is a key barrier to participation.
“Rare disease research has historically been very challenging, but new solutions are now available to make participation easier” says Joyce Moore, head of rare disease solutions at THREAD, a leading provider of decentralised clinical trial technology that has supported over 50 global rare disease trials. “The number of patients available with a rare disease is inherently small, and requirements for frequent long-distance travel to research clinics may discourage many otherwise motivated patients. When you consider that 50% of rare disease patients are children, this adds additional complexities.”

