SPONSORED BY Kyowa Kirin
The coronavirus pandemic is continuing to have a significant impact on healthcare systems and the industry’s ability to provide timely access to therapies for patients who need them.
Limited patient populations, lag in diagnosis and complex genetic tangles make drug discovery and provision an article of faith, as well as a scientific mission, in the rare disease area.
Kyowa Kirin, the Japanese innovative biotechnology and pharmaceutical company, follows a focused discovery pathway based on excellence in clinical analysis, genetic engineering and manufacturing technology. For more than 30 years, they have pursued scientific discoveries that help address unmet medical needs.
Kyowa Kirin, which is headquartered in Japan but has a strong UK and European presence, has five research facilities around the world that have created more than 50 therapeutic products for people living with a range of rare and more common conditions.
Kyowa Kirin has 18 drugs in development and will be launching three global products across its northern cluster, including the UK, Ireland, the Nordics and Baltics, over the next year.
With cutting-edge technologies and systemic therapies, the company is providing hope to people living with rare diseases, including rare cancers.
Although a rare disease is classed as affecting fewer than one in 2,000 people, there are 6,000 different types impacting around 3.5 million people in the UK and 30 million in Europe.6
“The rare disease area is particularly challenging, but commitment to life is our founding principle and it flows through everything we do. We never lose sight of patients, their families, carers and healthcare professionals, not to forget our teams,” says Richard Johnson, Kyowa Kirin’s northern cluster general manager.
“By definition, each rare disease affects very few patients but everyone of them is important to us. We aim to make a difference to their lives. We seek to understand the impact these conditions have on them through our engagement with the clinical and patient advocacy communities. We are constantly thinking about what they are experiencing and doing our best to meet their needs.”
Kyowa Kirin’s attention to the needs of people living with rare diseases is characterised by its holistic approach, which includes providing nurse homecare services, along with education and advocacy programme. The company’s objective is to optimise patients’ access to the right healthcare support and to increase patient groups’ voice in the health policy debate.
On average, it takes more than four years to receive an accurate diagnosis of a rare disease and Kyowa Kirin is committed to reducing this distressing time lag.6
“Our innovation is focused around unmet needs and improving the outlook for patients, and bolstering the ability of healthcare providers and professionals to offer effective treatments,” says Johnson. “We involve patient groups early in the process. They are the best placed to provide us with insights as to what will work best for patients.
“On the immediate horizon, we will be launching three global products across the northern cluster and have more products coming through clinical trials. It enthuses all of us to know we can make a difference to people’s lives and these are exciting times for us and people living with rare diseases.”
The company has four key pillars to its drug development programme: next-generation therapeutic antibodies, new small molecule drugs, nucleic acid drugs and regenerative medicine. It collaborates strongly with biotechs to advance innovative products.
A prime example of its approach has been recently gaining an expanded licence for a treatment for adults with a rare metabolic bone disorder, which previously had no systemic approved therapy.
Dr Robert Chipperfield, the company’s northern cluster medical lead, believes Kyowa Kirin’s corporate culture is sympathetically tuned to improving the lives of people living with rare diseases.
“You have to be dedicated to develop a drug for a rare disease as you are not going to be producing a blockbuster because the patient populations are small. But the unmet need is there. The satisfaction of helping people living with often lifelong and debilitating conditions to have a better quality life, to be able to enjoy their families, play with their grandkids, is hugely motivating and rewarding,” he says.
The satisfaction of helping people living with often lifelong and debilitating conditions to have a better quality life is hugely motivating and rewarding
“We make more than medicines. Our culture is to bring a smile to the faces of patients and that is very refreshing. We have ambition across rare diseases and oncology, and I’m excited about what the future holds for people affected by those conditions we are researching treatment for, for the company and our teams.”
Maintaining its core qualities and patient engagement has presented challenges during the pandemic, but Kyowa Kirin has adapted its processes to stay connected to all its stakeholders, including patient groups, clinicians, payers and healthcare providers, while continuing its fast-paced growth and innovation. Objective: discover medicines that have the potential to change lives.
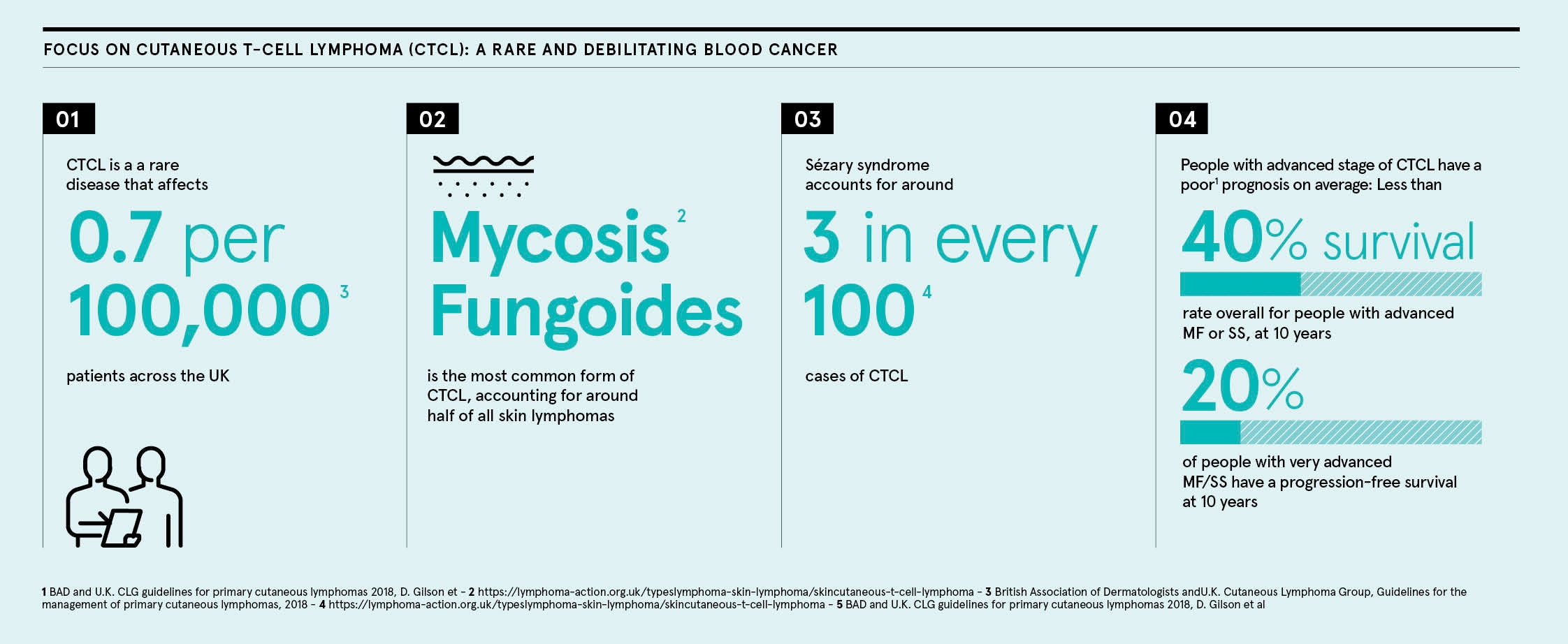
Rare disease focus: Cutaneous T-Cell Lymphoma (CTCL)
Rare diseases are often chronic and life threatening, and all exact an extreme toll on a patient’s mental health as, on average, each will receive three misdiagnoses, visit five doctors and wait four years before receiving an accurate diagnosis.7
Cutaneous T-cell lymphoma (CTCL) is a rare blood cancer that manifests on the skin with symptoms similar to psoriasis and eczema, which makes it difficult to spot and condemns patients to long periods of severe discomfort and distress.
“CTCL takes away all my energy; it eats me from the inside,” one patient says. “I no longer slept, my skin itched constantly. I was incapable of doing professional or intellectual work. It impacted our relationship as a couple.”
The condition is treatable, though not curable, but a key component in easing the burden is raising awareness and improving medical education so diagnoses can be delivered swiftly.
“A GP may only see one patient in their lifetime and, because it appears as skin lesions, patients can get stuck in dermatology conditions until someone puts two and two together,” says Chipperfield. “That delay in diagnosis can have a massive impact on quality of life, both for people living with the condition and their carer or carers, and on life expectancy. Only around half of patients (52%) with advanced MF/SS survive for 5 years.8 This is something that we and the healthcare community should tackle.
“We are committed to raising awareness and enhancing medical knowledge across the clinical journey so we can put the disease into remission and improve quality of life.”
Further information on CTCL can be found at lymphoma-action.org.uk and lymphomacoalition.org/Europe, clfoundation.org
6 https://www.raredisease.org.uk/what-is-a-rare-disease/
7 https://www.raredisease.org.uk/our-work/illuminating-the-rare-reality-2019/
8 Scarisbrick JJ, Prince M, Vermeer MH, et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol. 2015;33(32):3766-3773

