When James P. Allison of the United States and Tasuku Honjo from Japan won this year’s Nobel prize for medicine, the Nobel committee hailed their work as “an entirely new principle for cancer therapy”, which has fundamentally changed the outcome for some advanced cancer patients.
The two Nobel laureates’ research led to the development of a form of immunotherapy called checkpoint inhibitors. These release the brakes on the immune system, blocking proteins that stop it from attacking cancer cells.
They act on T-cells, white blood cells that can identify and kill cancerous cells; a single T-cell can kill thousands of cancer cells. T-cells have proteins that turn on an immune response and other proteins that turn them off. These are called checkpoints.
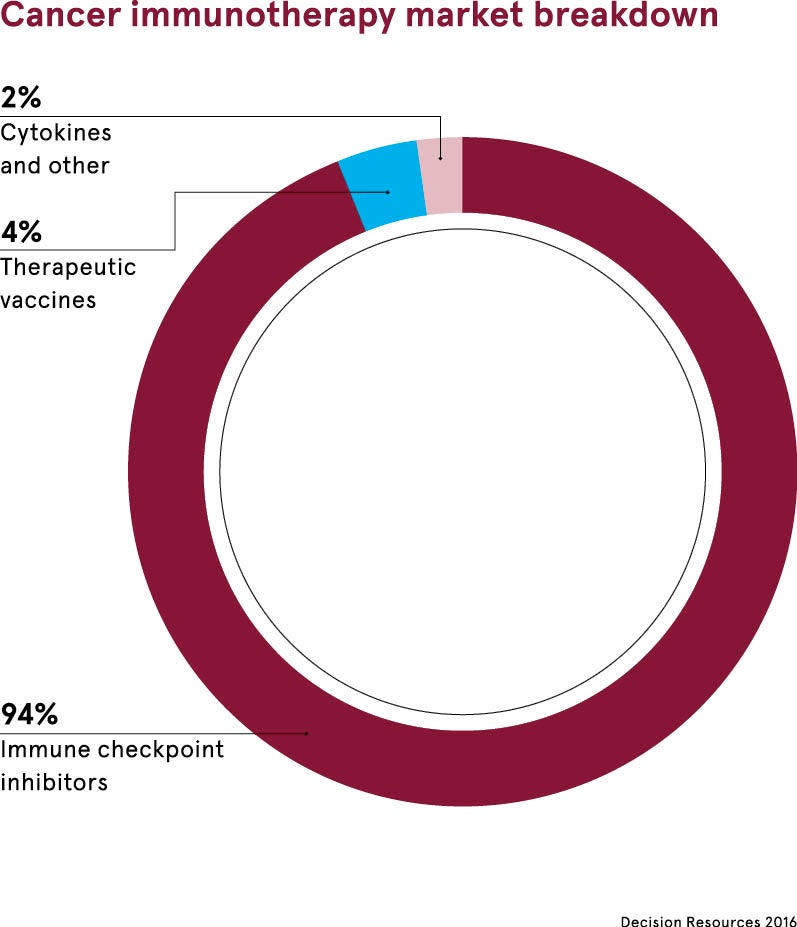
What do checkpoint inhibitors do?
Cancer Research UK explains: “Some checkpoint proteins help T-cells to become active; for example, when an infection is present. But if T-cells are active for too long or react to things they shouldn’t, they can start to destroy healthy cells and tissues. So other checkpoints help to tell T-cells to switch off.
“Some cancer cells make high levels of proteins. These can switch off T-cells when they should really be attacking the cancer cells. So the cancer cells are pushing a ‘stop button’ on the immune system; the T-cells can no longer recognise and kill cancer cells.”
Also known as monoclonal antibodies, checkpoint inhibitors stop the cancer cell proteins from pushing the stop button. This switches the immune system back on, enabling T-cells to resume their search-and-destroy mission.
The Nobel laureates succeeded where others had failed by working out the interaction between cells and fine-tuning the immune system.
What Allison and Honjo’s work has helped to do
The bearded, jovial, 70-year-old, harmonica-playing Dr Allison, chair of immunology at the University of Texas, developed an antibody to block a checkpoint protein called CTLA-4. This led to the first of the checkpoint inhibitors. Licensed in the US in 2011, ipilimumab became the first treatment to extend survival of patients with late-stage melanoma skin cancer.
Follow up studies showed that 20 per cent of patients lived for at least three years, but many survived for ten years or more – a spectacular result.
Dr Allison says: “I never dreamt my research would take the direction it has. It’s a great emotional privilege to meet cancer patients who’ve been successfully treated with immune checkpoint blockade. They are living proof of the power of basic science, of following our urge to learn and understand how things work.“
The Nobel laureates’ work also helped to establish treatment for lung, kidney, bladder, gastric, liver, cervical, colorectal, head and neck cancers, and Hodgkin’s lymphoma, a cancer in the lymphatic system, which helps to drain fluid and waste products from the body.
Checkpoint inhibitors do not work in every case
But while between 20 and 30 per cent of cancer patients respond extremely well to checkpoint inhibitors, most do not. Drug companies have been trying to increase success rates with two-drug cocktails. Worryingly, some of these combinations are said to be based more on guesswork and crossed fingers than good science.
Dr Allison explains: “One challenge is that the clinical success has outrun our scientific knowledge of how these drugs work and how they might best be combined with other therapies to improve treatment and reduce unwanted side effects.”
Side effects can be severe because checkpoint inhibitors boost all immune cells, not just the ones that target cancer. Overactive T-cells can generate nausea, skin problems, appetite loss, diarrhoea, breathlessness and a dry cough. Checkpoint inhibitors can also disrupt the liver, kidneys and hormone-making glands such as the thyroid.
It’s a great emotional privilege to meet cancer patients who’ve been successfully treated with immune checkpoint blockade
Unfortunately, combination therapy, such as ipilimumab and another checkpoint inhibitor nivolumab, has been shown to exacerbate side effects in patients with advanced melanoma.
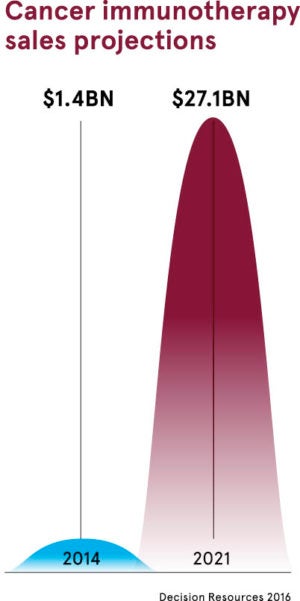
Big Pharma competing to make checkpoint inhibitors profitable
One combination that has been conclusively proven to work involves twinning a checkpoint inhibitor with chemotherapy, a mainstay of cancer treatment for more than 70 years. But chemotherapy is also associated with debilitating side effects such as hair loss and vomiting.
All this explains why more than 1,500 treatment trials are underway in what is, in essence, one of the biggest ever games of pharmaceutical roulette. The potential financial rewards for Big Pharma are breathtaking as a winning treatment could generate, in theory, hundreds of millions of dollars of profit. But according to one recent estimate, widespread use of immunotherapy agents in the US alone could cost $174 billion.
Costs in the UK are substantially less than across the Atlantic. For example, pembrolizumab, which can extend the life of some patients with lung cancer for a year or more, has a full list price of £84,000 a year, but the manufacturers Merck, Sharp and Dohme have given the NHS a confidential discounted price. This followed a trial in which it was shown to extend life by 16 months more than standard chemotherapy. About 1,800 UK patients a year are eligible for treatment.
The more successful the new treatments become, the harder it will be to juggle demand with resources. Deloitte’s, specialists in tax and risk management, recently reported that it costs the world’s top bio-pharma companies more than $2 billion to bring a drug to market.

What do checkpoint inhibitors do?
What Allison and Honjo's work has helped to do