 Since its creation in 1980, Amgen has been a pioneer in the science of using living cells to make biologic medicines. In less than 40 years, it has grown from a single building in California to a worldwide leader in biotechnology and the seventh largest pharmaceutical company in the world by market capitalisation. Today it has a valuable portfolio of therapies to treat cancer, cardiovascular, bone and kidney disease.
Since its creation in 1980, Amgen has been a pioneer in the science of using living cells to make biologic medicines. In less than 40 years, it has grown from a single building in California to a worldwide leader in biotechnology and the seventh largest pharmaceutical company in the world by market capitalisation. Today it has a valuable portfolio of therapies to treat cancer, cardiovascular, bone and kidney disease.
Key to Amgen’s success has been its approach to research and development. With a focus on treatments for patients with the most serious illnesses, it is using advanced human genetics to shed new light on the molecular roots of many diseases.
These medical advances are coming though at a time when healthcare budgets are increasingly strained, the result of ageing populations and a rise in the number of people living with chronic diseases including cancer and dementia. How healthcare systems reconcile these increasing demands for care and benefit from this new wave of medical innovation is a significant issue for us all.
According to John Kearney, managing director for Amgen UK and Ireland: “The challenge facing healthcare systems and companies like Amgen is how to work together so patients can have quick and consistent access to new medicines that help them live longer and healthier lives. This also includes finding more efficient and effective ways of delivering healthcare”.
Amgen has been at the forefront of companies that are working with local NHS organisations in England to enhance the delivery of patient care, with over 30 active projects that directly impact more than 15,000 NHS patients.
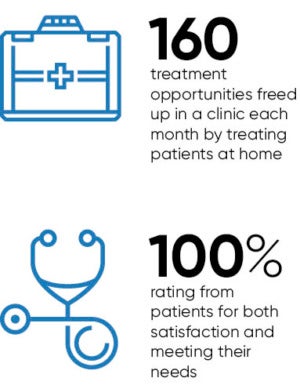
The multi-award winning project with the Clatterbridge Cancer Centre NHS Foundation has resulted in more patients being treated closer to home, reducing their travelling times and costs as well as improving patient satisfaction with their treatment. It also saved the NHS money and freed up important space on the cancer ward.
While local NHS organisations have been quick to work with companies like Amgen, Mr Kearney believes that “more needs to be done by NHS leaders to speed up and replicate these new and best practices across England”.
He also believes that the fragmented nature of decision-making in England works against the quick and consistent use of new medicines across the country. “We agree the price of new medicines with the Department of Health, NICE [National Institute for Health and Care Excellence] makes recommendations on the use of new medicines to the NHS, while the NHS manages the healthcare budget. The reality is that each of these organisations is working to a different agenda so we lack a coherent approach to the introduction to new medicines,” says Mr Kearney.
“I believe we need a new strategic partnership with government, NICE and the NHS to answer the conundrum on how we accelerate the use of innovative new medicines while balancing the need for the NHS to work within its budget. In England, this would create a singular agreement about the pricing, introduction and use of new medicines.”
Amgen are encouraged that last year prime minister Theresa May launched an industrial strategy and the life science sector remains one of its priorities.
As a company which has developed from a single building in California less than forty years ago, Mr Kearney’s message to government from Amgen is clear: “No matter how much you do to help a company discover, develop and make new medicines, this time and investment will be wasted if healthcare systems do not enable patients to have quick and consistent access to these new medicines.”
For more information please visit www.amgen.co.uk


